New Rare Disease Dataset Just Dropped
Focus on life-changing research, not curating and cleaning data.

At any given time, millions of people (3.5–5.9% of the global population) suffer from rare conditions without effective treatments or cures. Rare diseases, therefore, present compelling opportunities for Drug Development. Since each disease is present in small groups of patients, this research is also fraught with complex challenges. Limited numbers of patients translate to scarce information, making this research risky and expensive.
Regulatory agencies such as the FDA have recognized these challenges and have created programs to support this research through regulatory and financial incentives. In the United States, the Orphan Drug Act has provided a framework for this support since 1983 by issuing orphan designations to drugs being investigated for treating a rare disease.
We are excited to bring FDA Orphan data into DrugBank because we know that at the earliest stages of drug discovery, curating reliable scientific data, identifying relevant previous trials, and designing protocols for success are all important, challenging, and time-consuming tasks.
We’ve heard that while these steps are crucial and unavoidable, it can also feel as if they’re standing in the way of doing the research you set out to do. To alleviate this pain we're working to support you and your research by doing what we do best: curating, authoring, and maintaining clean, well-connected, quality drug data. Our team focuses on getting you the data you need so you can jump right into the life-changing research you want to be doing, instead of using your precious time on data-cleaning.
We're excited to expand our data offering by launching a new Rare Disease add-on to complement our Clinical Trial dataset. This data will enable you to move faster and do more with DrugBank. This new resource for rare disease research will be integral to our overall drug development solution.
It includes
- Standardized trial termination reasons
- FDA Orphan designation information
- Structured drugs and rare conditions
And can be added to our existing clinical trials dataset. Which now includes eligibility criteria data!
The result is a more refined source of competitive and scientific data. With a greater understanding of what prior trials were conducted for a drug or disease, you can gain powerful insights to help:
- Prioritize disease areas
- Build a case for leveraging regulatory incentives
- Identify drug repurposing opportunities
- Optimize study designs
For this release, our medical experts spent more than 1,000 hours curating essential information and establishing meaningful connections between concepts resulting in thousands of conditions, standardized trial termination reasons, and connected drugs so that you can leverage this data for insights. And we’re not done yet, our team will continue to curate and maintain this vital information, with more exciting features already underway.
To illustrate, here is a two-part example of the kind of analysis you could perform with this data:
Identify opportunities
With the termination reason feature, you can search for a drug and see metrics that could empower your decision-making. As an example, you will be better able to understand your competitors' mistakes and more easily identify opportunities for drug or trial optimization.
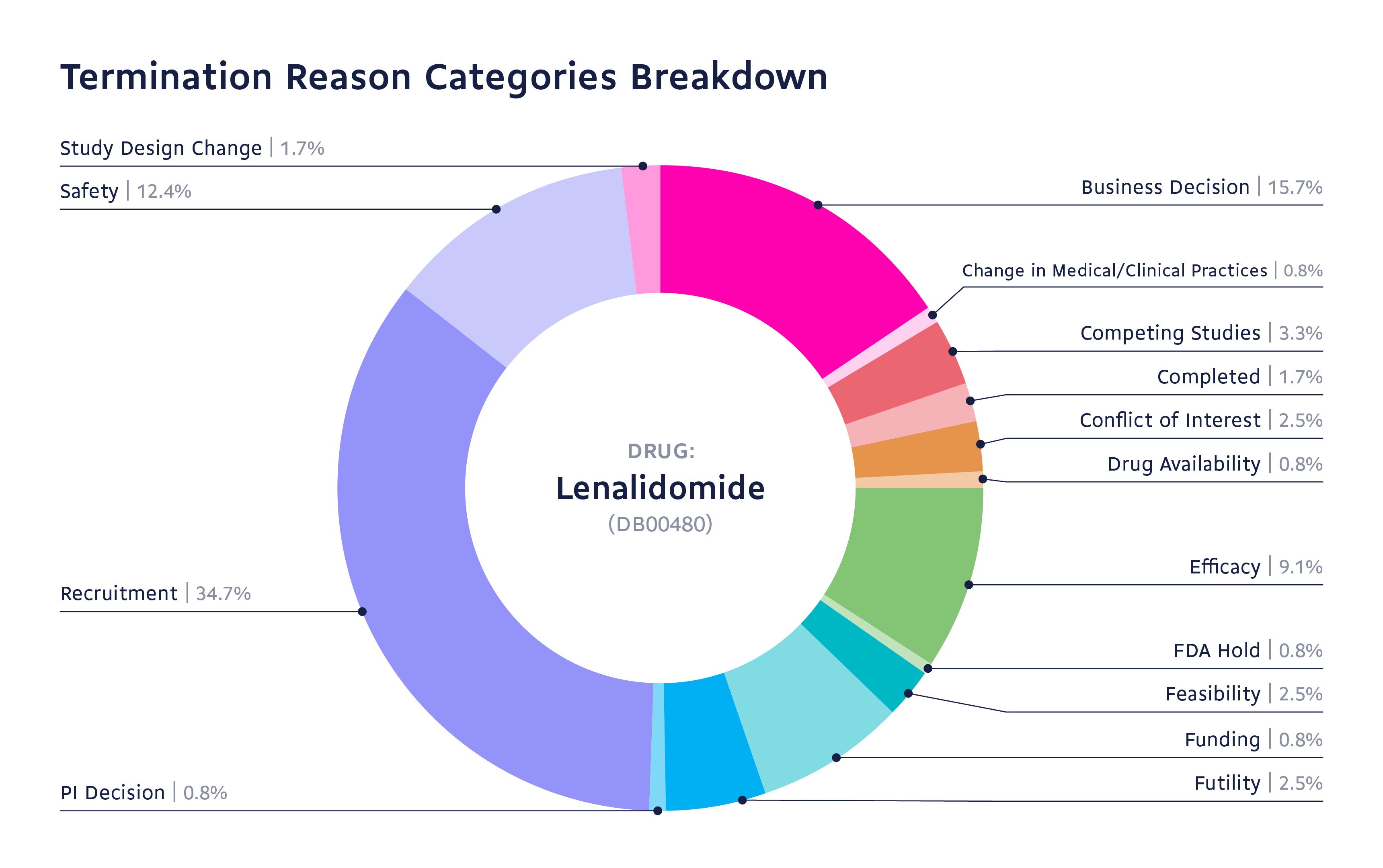
Explore trial termination reasons
Let’s say you were interested in the drug Lenalidomide. Lenalidomide is indicated for adult patients with multiple myeloma, myelodysplastic syndromes, mantle cell lymphoma, follicular lymphoma, and marginal zone lymphoma in selected patients. At a glance, you can understand why trials on this drug were terminated and make informed decisions based on this information:
Examine orphan designations
Within the Rare disease add on and for the same drug, you can now also cross reference for information and designations from the FDA Orphan Products Program. This list of orphan designations provides strategic insights when planning your pipeline.
Insights include the exclusivity end date for each orphan designation
We work hard at DrugBank to continually add new and improved data and datasets to make it easier for researchers to work with speed and accuracy. The addition of rare disease clinical trial data opens up even greater opportunities for advancements and deeper insights far beyond the few examples we’ve included here.
Our commitment to quality data means that we won’t stop improving our data, the scope of information, and the depth of our connections. With that in mind, stay tuned for another announcement about more enhancements that are on their way.
Contact our sales team to find out more about this research package.

References
- Evaluate Pharma Orphan Drug Report 2020
- NIH Genetic and Rare Diseases Information Center - Waldenström macroglobulinemia
- DrugBank Online - Lenalidomide



